What to Tell Your Patients this Flu Season
Posted by Emily Thompson on Sep 1st 2020
As a pharmacist you know you are on the front line of the annual flu season because you interact with patients more often than anyone else in the medical care chain. You also know that the best way to do your part to enhance public health and reduce an individual’s risk of contracting the flu is to encourage all to get the annual vaccine.
This season you have your work cut out for you.
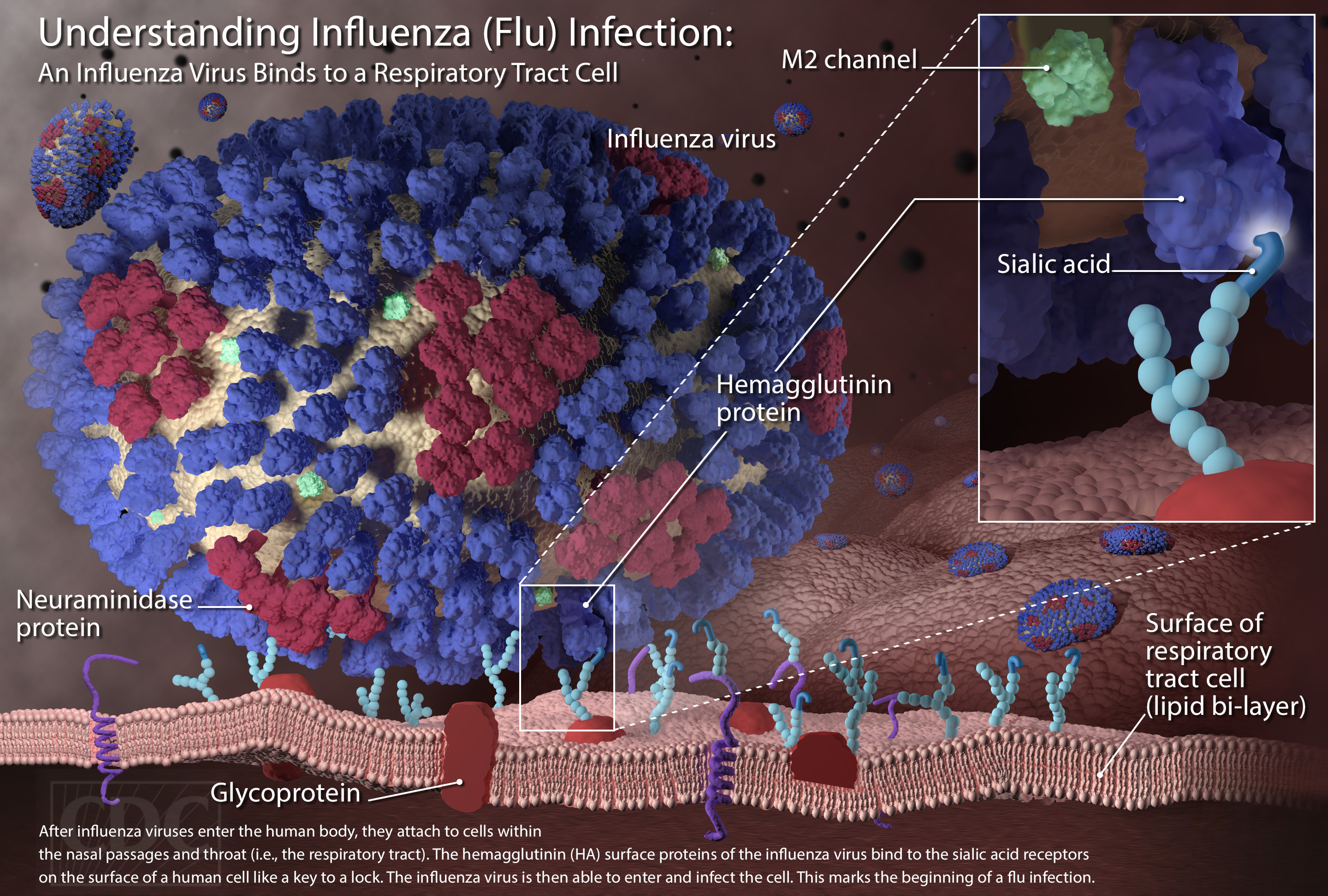
Understanding The Challenge
What is going to be so tough this year, without even yet knowing the severity of the coming season, is convincing a skeptical public that getting the vaccine is worth it.
In March, Dr. Lisa Grohskopf of the Centers for Disease Control and Prevention told the New York Times the 2014-2015 influenza vaccine was only about 18 percent effective, much less than half of expected efficacy, against that season’s Type A H3N2 strain. More so than the other flu types, Type A is most responsible for bringing about the onset of pneumonia and other complications and is especially risky to people aged 65 and above and young children.
What happened was a mismatch between which Type A strain the vaccine targeted and the so-called drifted strain that actually emerged. By the time the drifted strain was properly identified last September as being the dominant strain, the season’s 140 million doses of vaccine had already been produced and shipped.
It was the fourth time in 20 years there had been an important mismatch. But if you flip that script, that does mean that influenza vaccines are properly matched 80 percent of the time.
Then in April more bad news came. GlaxoSmithKline sent an urgent vaccine recall letter to about 1,000 of its customers urging them to stop distributing, immediately quarantine, and send back all remaining units of its very popular Flulaval Quadrivalent Thimerosal-free vaccine Pre-Filled Syringes. According to the New York Times the recall involved some 1.7 million doses.
The quadrivalent vaccine is part of the new group of injectable and inhalable immunization products that address four strains: two for Type A flu and two for Type B flu. Traditional vaccines protect against three strains: two Type A strains and only one Type B strain.
It was widely reported that reason for the recall, as stated in the urgent letter, was that the company had, “observed loss of potency below the minimum specification prior to product expiry for the B strains included in the vaccine.” And, that it, “cannot rule out potential suboptimal protection for product administered from early January 2015 and beyond.”
And lastly there are fears that the vaccine is more dangerous than the disease. The flu vaccine has been caught up in the debate over the safety of the measles vaccine, which some strongly believe is connected to a child’s development of autism. While this assertion has been debunked by numerous scientific studies, The Boston Globe reported that parents in well-educated and well-off communities have been refusing to allow their children to receive vaccines.
What to Tell Your Patients, a Q & A Refresher
Q: Looking at what happened last year, isn’t the flu vaccine ineffective?
A: Last year’s flu vaccine was not the best, but in the past 20 years the vaccine has been effective for 80 percent of those years.
Q: Is the flu really an epidemic?
A: Yes. According to the CDC on average 5 to 20 percent of the U.S. population gets the flu and more than 200,000 people are hospitalized from seasonal flu-related complications.
Q: Is the flu deadly?
A: Most definitely. Every year on average 24,000 die from the flu in the United States. The Centers for Disease Control considers the flu to be one of the deadliest infectious diseases, as it has caused as many as 49,000 deaths in one year since records began in 1976.
Q: Does this epidemic really occur every year?
A: The United States experiences a flu epidemic every year. The epidemic is seasonal, starting in October and ending in May, and is at its worst between December and February.
Q: Do I really need to get vaccinated?
A: While most people who do fall ill due to the flu recover without needing formal medical care, most people do not live and work in isolation. Playing your part to curb an epidemic means not making those with whom we come into contact, who may be more at risk of serious complications, ill.
Q: Who is at risk of serious complications?
A: You likely know someone who is at risk of serious complications. Do you know someone over the age of 65; a child under the age of five; a pregnant woman or a woman who recently gave birth; a person who lives in a nursing home; a Native American; or someone who suffers from heart disease, metabolic disorders including diabetes, or obesity? They are all at risk.
And if you really want to drive this point home and depending on your audience:
- For the 2014-2015 flu season, almost 94 percent of all adults hospitalized for the flu had at least one underlying medical condition.
- Children under the age of five have the second highest hospitalization rate. For the 2014-2015 flu season for every 100,000 children aged under five, 58 were hospitalized with the flu. It is estimated that on average 20,000 young children are hospitalized each year because of the flu.
- Also for the last season, for every 100,000 seniors, 322 were hospitalized, a record since this type of record keeping began. The previous record from two seasons ago was 183 per 100,000 aged 65 and above.
More information on who is at risk and which health conditions put a person at risk can be found here on the CDC website.
Q: How long will the vaccine last?
A: The vaccine should last at least six months, but can be good for eight months and even longer.
Q: Who should get vaccinated?
A: It is recommended that everyone 6 months of age and older should get vaccinated once the vaccine becomes available and if at all possible, before October. Even if you wait until after October, get vaccinated anyway.
To drive home the point about the necessity of vaccinating children:
- In the 2012-2013 flu season, 90 percent of all the children under 18 who died because of the flu, were not vaccinated.
Please note the CDC says that for some children aged 6 months to 8 years may require two doses of vaccine. This is especially true for children receiving the vaccine for the first time. A physician should be consulted.
Q: What about children under 6 months?
A: They are too young. The best way to protect them is to get yourself vaccinated and ensure they do not come into contact with anyone who may have been exposed to the flu.
What’s New This Year?
The CDC-approved 2015-2016 influenza vaccine is made to protect against the following three viruses:
- an A/California/7/2009 (H1N1)pdm09-like virus
- an A/Switzerland/9715293/2013 (H3N2)-like virus
- a B/Phuket/3073/2013-like virus. (This is a B/Yamagata lineage virus)
The 2015-2016 quadrivalent flu vaccine also protects against an additional B virus (B/Brisbane/60/2008-like virus). This is a B/Victoria lineage virus.
Here is the CDC’s complete list of approved flu vaccines and recommendations concerning age.
Photo courtesy of the CDC
